📌 Key Takeaways
A supplier’s “FDA compliant” label means nothing if the test conditions don’t match how you actually use the packaging.
- Match Tests to Your Menu: Migration testing only proves safety for the exact food type, temperature, and hold time that was tested—not all uses.
- Check Six Key Lines: Look for regulation name, test method, food simulant, time/temperature, units, and actual results with limits on every TDS.
- Old Documents Hide Risk: A TDS older than 12–24 months may reflect ingredients or coatings the supplier no longer uses.
- Units Aren’t Interchangeable: mg/kg (per food weight) and mg/dm² (per surface area) measure different things and can’t be compared directly.
- “Complies” Isn’t Proof: A TDS that says “complies” without showing the actual test result and limit won’t survive an audit or inspection.
Test conditions prove compliance—not labels or claims.
Food service operators and procurement managers sourcing packaging for hot, greasy, or acidic menu items will find a ready-to-use verification checklist below, preparing them for supplier conversations that follow.
~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~
A supplier’s technical data sheet lands in your inbox with “FDA compliant” printed near the top. The document looks official. But when a health inspector asks how the test conditions match your hot, greasy menu items, that confident label suddenly feels like a question you cannot answer.
The objective is to confirm that a supplier’s evidence matches the way the food packaging paper is actually used—hot, greasy, acidic, frozen, microwaved, or held for hours.
This guide explains how to read the migration testing section of a Technical Data Sheet (TDS) like audit evidence–not marketing material. This analysis determines whether the test conditions, limits, and scope actually apply to your menu and operations.
What Migration Testing Means (and What It Doesn’t)
Migration testing measures whether chemicals transfer from food packaging paper into food under specified conditions, and whether that transfer stays within defined regulatory limits.
Two categories appear on most technical data sheets. Overall migration measures the total mass of all substances that transfer, regardless of what they are–think of it as a ceiling check. Specific Migration Limits (SML) track individual substances, particular chemicals with their own safety thresholds. A material can pass the overall migration test yet fail an SML for a coating additive.
For a detailed breakdown of SML compliance workflows, see navigating specific migration limits (SML).
A claim like “food safe” or “compliant” tells you nothing about whether the testing conditions match your actual use. A wrapper tested at room temperature for dry pastries provides no evidence for burgers held at 160°F (~70°C) in a warming drawer—a mismatch that the Menu-Match Matrix for food packaging paper helps you avoid by classifying items by heat, grease, and hold time. The test conditions and limits–not the label–determine whether the evidence applies.
Confirm the TDS Is Current and Actually About Your Grade
Before examining test results, verify the document matches the product you are purchasing.
Grade and SKU: The TDS should name the exact product, not a product family. This specificity aligns with the specifications-first protocol that prevents generic sourcing failures. “Greaseproof liner series” is too vague; “GP-2400 coated sandwich wrap” is specific.
Coating or barrier: If your paper has a grease-resistant treatment—such as a greaseproof paper coating—the TDS must specify that coating. Data for uncoated paper does not apply to coated products—and vice versa. For guidance on selecting the appropriate barrier type, see poly-coated vs. uncoated: choosing the right barrier for hot and steamy foods. Recycled and virgin pulp also carry different migration profiles. See recycled vs. virgin pulp: understanding migration risks.
Revision date: Verify the version number. Documents older than 24 months often reflect obsolete formulations. Suppliers reformulate coatings and change raw material sources; any change can invalidate prior results.
Document Hierarchy: A TDS summarizes typical properties, whereas a Certificate of Analysis (CoA) provides batch-specific results and a Letter of Guarantee (LoG) serves as the formal compliance statement. For the complete document set buyers should maintain, see food packaging paper trail: 3 documents you need to pass a health inspection.
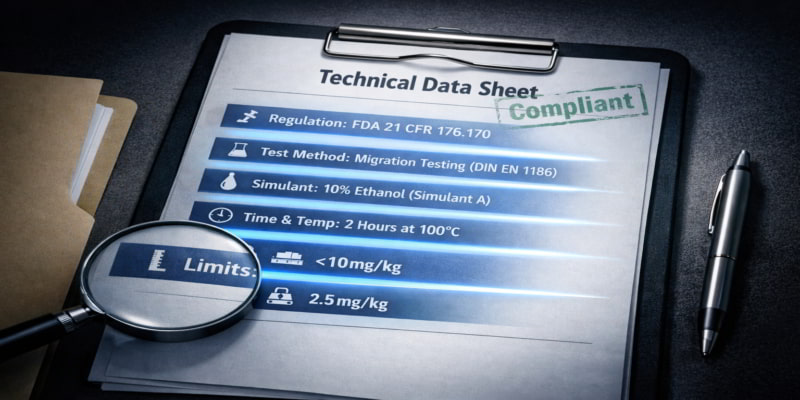
The 6 TDS Lines That Decide Whether a Migration Result Is Usable
Once identity and currency are confirmed, examine the migration section for six elements:
| What to Verify | |
| 1. Regulation scope | Which market’s rules? US (FDA 21 CFR 176.170), EU (EC 1935/2004), or other. |
| 2. Test method | Named standard (e.g., EN 14338 for paper/board, EN 1186 for plastic coatings, or FDA extraction methods). No method = no verification. |
| 3. Food simulants | Simulant A (aqueous), B (acidic), D (fatty/oil), E (dry). Your food type dictates which matters. |
| 4. Time/temperature | Exact conditions: “2 hours at 70C.” Room-temperature tests do not cover hot-holding. |
| 5. Units | mg/kg (per kg of food) or mg/dm2 (per surface area). Not interchangeable. |
| 6. Limits and results | Both the regulatory limit and actual result should appear. “Complies” alone is not verifiable. |
For U.S. paper components, the regulatory framework is detailed in 21 CFR 176.170.
The absence of any single element reduces the migration section to a summary, voiding its utility as audit-grade evidence.
Simulants, Thermal Exposure, and Metric Standardization
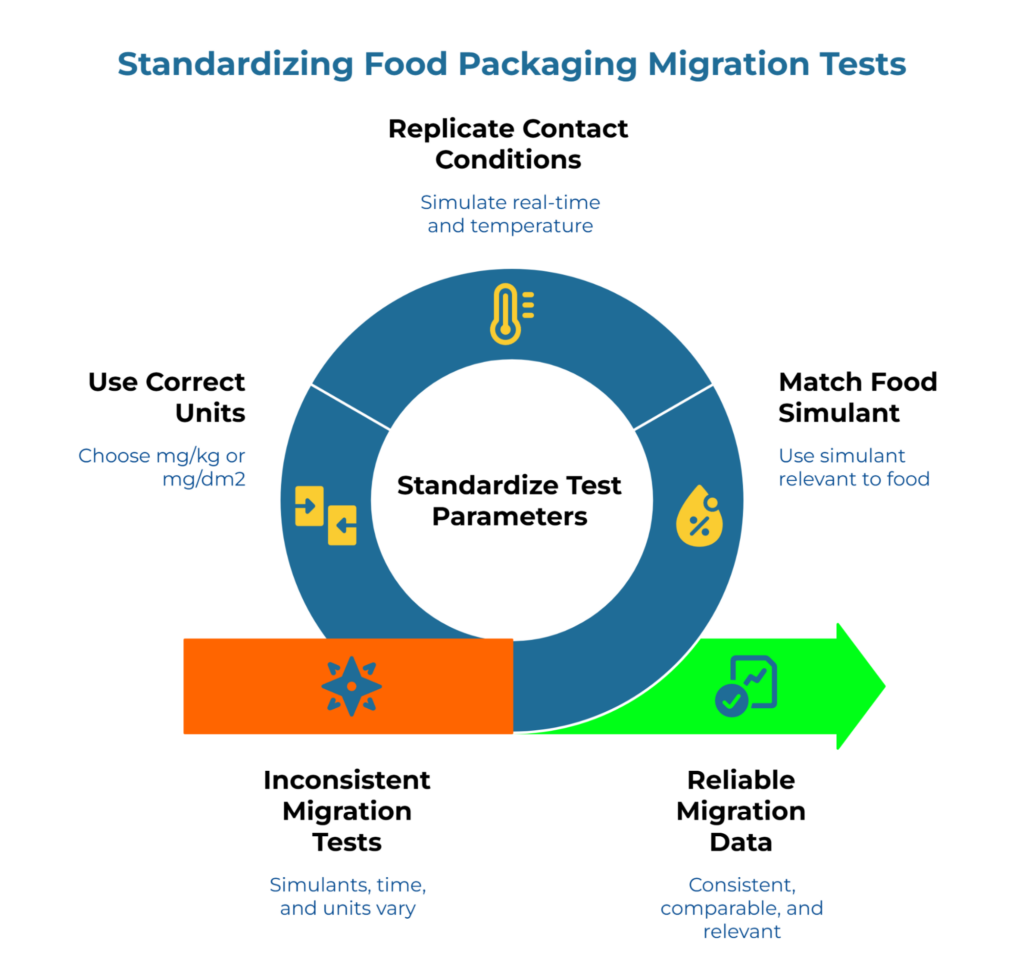
Food simulants are standardized liquids that mimic how different food types interact with food packaging paper. Simulant D (vegetable oil) represents fatty foods like burgers. Simulant A (water-based) represents soups or sauces. The simulant must match your food category for the test to be relevant.
Time and temperature replicate real contact conditions. A test at 105°F (~40°C) for 10 days simulates long-term ambient storage. A test at 160°F (~70°C) for 2 hours simulates hot food contact—the same thermal range addressed in the wax paper trap: why generic wraps fail high-heat tests, which explains why coating behavior changes at elevated temperatures. If your operation holds food at 160°F (~70°C) for 45 minutes, ensure the test parameters encompass or surpass these specific thermal limits.
Units matter significantly. mg/kg measures migration per kilogram of food–useful when you know the food-to-packaging paper ratio. mg/dm2 measures migration per square decimeter of material surface–useful for comparing materials directly.
Converting between these units requires assumptions about how much food contacts how much food packaging paper surface–assumptions that may not match real service conditions. These units are not interchangeable, and mixing them leads to false comparisons.
Mapping Test Conditions to Operational Reality
Create a quick profile of your use case by answering four questions:
Food type—fatty (burgers, fries), aqueous (soups), acidic (tomato-based), or dry (bread)? For fatty foods, understanding Kit levels for grease resistance helps translate food type into specification requirements.
Temperature–hot off the grill, warm-held, room temperature, or refrigerated?
Contact time–minutes, hours, or days?
Reheating–will customers microwave or oven-reheat in the food packaging?
Compare your profile to the TDS test conditions. Look for the closest match. If the TDS was tested at 105°F (~40°C) for 2 hours but you hot-hold it at 160°F (~70°C) for 45 minutes, there is a gap. Request addendum testing from the supplier, or find a supplier with data that matches your conditions.
For an evidence-first framing of certificates versus ongoing verification, see the compliance shield: how to audit your food packaging paper suppliers.
Red Flags: When a TDS Looks Official but Fails as Evidence
Watch for these warning signs:
Missing test conditions–“Migration compliant” with no simulant, time, or temperature stated.
No laboratory identified–results without a named lab or accreditation reference.
Overbroad claims–“Suitable for all food contact applications” without specifying which regulations or conditions. As detailed in importing food packaging paper: how to verify international safety certifications, most certified imports fail because certificates cover a different plant or grade, not because paperwork is missing.
Results without limits–showing only the measured value, not the threshold it is compared against.
Grade mismatch–TDS covers a product family but does not specify your exact SKU or coating.
A “food safe” claim with no named scope, test method, or conditions is not evidence by itself. See why “food safe” is a meaningless label for more on this distinction.
These gaps disqualify the document during formal compliance reviews or health inspections.
10-Minute TDS Triage Checklist
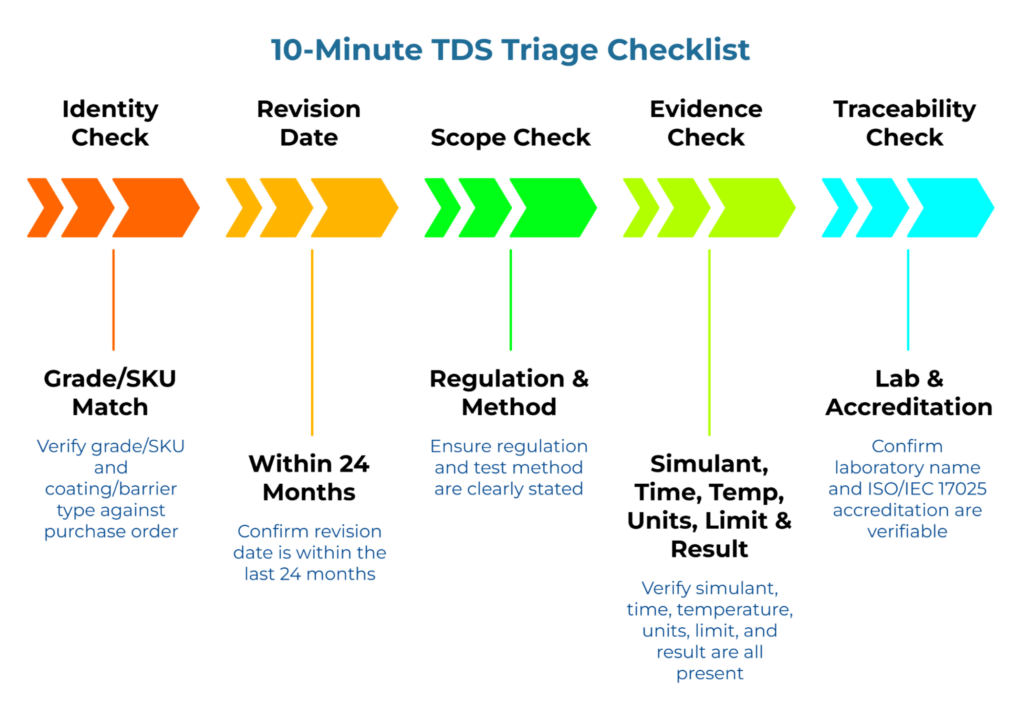
Use this checklist when evaluating any supplier’s TDS. Each gate should pass before proceeding.
| Check | Pass Criteria | |
| Identity | Grade/SKU matches purchase order | Exact match |
| Coating/barrier type specified | Named, not generic | |
| Revision date within 24 months | Date visible | |
| Scope | Regulation stated (FDA, EU, other) | Named regulation |
| Test method referenced | Appropriate EN/FDA method cited (e.g., EN 14338 or EN 645) | |
| Evidence | Simulant(s) named | Matches food type |
| Time and temperature stated | Matches use conditions | |
| Units stated (mg/kg or mg/dm2) | Explicit | |
| Limit AND result both shown | Both values present | |
| Traceability | Laboratory named | Identifiable facility |
| Lab accreditation (ISO/IEC 17025) | Verifiable |
File passing documents with the corresponding CoA and Letter of Guarantee for your audit records.
When the TDS fails at Gates 3-6, the practical next step is to source suppliers who can provide complete evidence packs. Find verified food packaging paper suppliers on PaperIndex or submit an RFQ to request quotes with current TDS and supporting evidence.
Supplier Verification Protocol
When the TDS passes triage but gaps remain, send these follow-up questions:
1. Can you provide the underlying lab report with the test report ID for these migration results?
2. Which regulatory framework does this testing satisfy–FDA, EU (EC) 1935/2004, or both?
3. Is the testing laboratory ISO/IEC 17025 accredited, and which accreditation body issued it?
4. Our application involves [describe: fatty foods, hot-holding temperature, contact duration per your menu-match matrix]. Does current testing cover these conditions?
5. Were tests performed on the finished article (including coatings and laminations), or only the base paper?
6. If current testing does not match our conditions, can you provide addendum testing? What is the timeline?
7. What is your revision cycle for TDS documents, and how will formulation changes be communicated?
8. Can you provide a Letter of Guarantee specific to our intended use conditions?
For context on how FDA and ISEGA certifications compare, see FDA vs ISEGA for takeout food packaging paper.
If your procurement workflow also requires tightening specifications so quotes are comparable and evidence is requested up front, how to create your mill spec sheet is the next logical tool.
Annotated Example: Reading a Migration Section
Below is a de-identified example showing how migration data typically appears on a TDS, with interpretation notes:
Case Study: Interpreting Migration Data
| Value | Interpretation | |
| Revision | Rev 4.2 — 03/15/2025 | Current document |
| Regulation | EU (EC) No 1935/2004 | EU scope–verify FDA if needed |
| Test methods | EN 14338 (or EN 1186-x cell method for coatings) | Named standard |
| Simulant | Simulant D2 (vegetable oil) | Fatty foods–appropriate for burgers |
| Conditions | 2 hours at 160oF (~70oC) | Hot contact–check against hold time |
| Overall migration | < 10 mg/dm2 (Limit: 10) | Result and limit shown |
| Laboratory | ABC Testing GmbH (ISO/IEC 17025) | Accredited–verify via ILAC |
This example shows a usable migration section: current revision, named regulation and test methods, specific simulant and conditions, result with limit, and an accredited laboratory. Accreditations can be verified through the ILAC MRA signatory database.
If the TDS stops at “defined conditions” without listing them, file it as a pointer–not final evidence–and request the report.
Frequently Asked Questions
What is the difference between overall migration and specific migration (SML)?
Overall migration quantifies the cumulative mass of all non-volatile substances transferred to the simulant. SML tracks individual chemicals with their own limits. A product can pass overall migration yet fail an SML for a particular additive.
Do uncoated papers need migration evidence?
Yes. All food-contact paper—coated or uncoated—can transfer substances from processing chemicals, sizing agents, or pulp. For a deeper understanding of how pulp source affects migration risk, see recycled vs. virgin pulp: understanding migration risks in food packaging. The absence of a coating does not eliminate the need for migration testing.
How often should a TDS be updated?
Generally, every 12-24 months or whenever formulations change—a timeline that aligns with the compliance decay patterns described in why food-safe labels fail: how compliance decay happens in food-grade packaging paper. Ask your supplier about their revision cycle and how they communicate changes.
Why do some results show mg/kg and others mg/dm2?
They describe different bases (food mass vs contact area). Converting between them requires assumptions about how much food contacts how much surface area, which can be highly use-case dependent.
Does “FDA compliant” on a TDS mean the food packaging paper is approved by FDA?
In the U.S., food-contact paper components are governed through regulatory frameworks like FDA’s indirect additive regulations for paper components. Broad ‘approved’ language is usually a signal to ask for the specific CFR citation and supporting documents—see the compliance shield: how to audit your food packaging paper suppliers for a four-gate verification protocol. Broad “approved” language is usually a signal to ask for the specific CFR citation and supporting documents. See 21 CFR 176.170 for the regulatory text.
How does EU-style documentation differ?
EU food-contact frameworks are often referenced through the EU framework regulation (EC 1935/2004) and, for paper and board, common reference points include documents like BfR recommendations.
What is the difference between FDA, ISEGA, and EU compliance?
FDA (US) and EU regulations are governmental frameworks. ISEGA is a testing and certification body that evaluates materials against EU or other standards. An ISEGA certificate indicates testing was performed–but the test conditions still need to match your use case. See FDA vs ISEGA for takeout food packaging paper for the full comparison.
Is a TDS sufficient for a comprehensive audit trail?
A TDS is a summary; comprehensive audits require traceable documentation including test report IDs and lot-linked declarations. Food packaging paper trail: 3 documents you need outlines common document sets buyers maintain.
Disclaimer:
This article is for informational purposes only and does not constitute legal, regulatory, or safety advice. Food-contact compliance depends on your product design, menu conditions, and applicable regulations. Consult qualified compliance professionals and your supplier’s documentation before making decisions.
Our Editorial Process:
Our expert team uses AI tools to help organize and structure our initial drafts. Every piece is then extensively rewritten, fact-checked, and enriched with first-hand insights and experiences by expert humans on our Insights Team to ensure accuracy and clarity.
About the PaperIndex Insights Team:
The PaperIndex Insights Team is our dedicated engine for synthesizing complex topics into clear, helpful guides. While our content is thoroughly reviewed for clarity and accuracy, it is for informational purposes and should not replace professional advice.